Potent gene knockdown in VIVO with the RNAi-cap
A. Introduction
Experimental gene knockdown in small animals is an important pre-requisite for validation of cell culture experiments in the fields of cancer research, degenerative diseases and metabolic diseases. Usually, after successful gene knockdown in cell culture, scientists move to experiments in small animals with the same siRNA or miRNA mimic so far used. Therefore, they order large scales of siRNA or miRNA mimic desalted and endotoxin free. Because RNA is degraded in body fluids within minutes (de Fougerolles et al., 2007) high amounts of siRNA or miRNA mimics are needed, in repeated applications.This results in two major problems: i) an increased toxicity in animals due to off-target effects and the burden of the RNA by itself that activates innate immunity (Kleinmann et al., 2008), and ii) the resulting high costs of the RNAi reagents.
Riboxx has developed a new technology, the RNAi-cap, especially dedicated for gene knockdown experiments in vivo. The RNAi-cap increases up to 3-fold siRNA and miRNA mimics resistance to serum degradation, and increases up to 5-fold the potency of gene knockdown. As a consequence, less siRNA and miRNA mimic is needed for efficient gene knockdown in vivo, resulting in decreased toxicity and lower costs of goods.
B. Results and discussion
B.1 The RNAi-cap increases serum resistance of siRNA
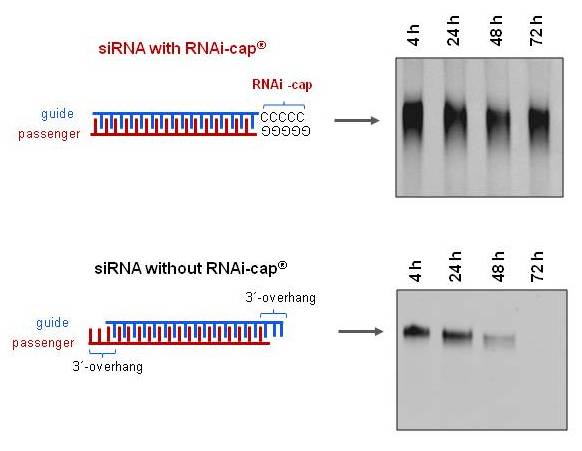
siRNA with or without the RNAi-cap were incubated in mouse serum up to 72h and its degradation visualized at defined time points (on native PAGE by UV transillumination. As shown in Figure 1, siRNA with the RNAi-cap were resistant to degradation up to 72 hours (3 days) in mouse serum. In contrast, siRNA without the RNAi-cap was degraded at 48h.
Fig. 1. The RNAi-cap confers serum resistance to the siRNA. siRNA were incubated at a concentration of 3 µM in mouse serum (80% v/v) at 37°C for 72 hours (3 days). At indicated time points, integrity of the RNA duplex was assessed by 20% native PAGE. Visualization was performed by UV transillumination after staining by GelRed.
B.2 The RNAi-cap increases gene knockdown potency up to a 5 fold
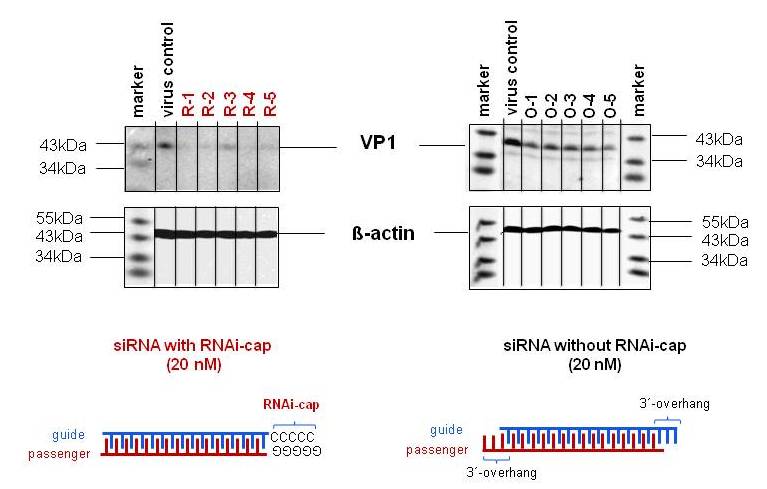
Five siRNAs targeting the viral genome of Human Rhinovirus Type1B where designed with or without the RNAi-cap. After infection of HeLa cells with HRV1B, the siRNAs were transfected at 20 nM concentration. As shown on Figure 2, none of the siRNA without the RNAi-cap could generate a gene knockdown at 20 nM. By adding the RNAi-cap to the siRNA, complete gene knockdown was evidenced.
Fig. 2. Increased potency of gene knockdown with the RNAi-cap technology. Five siRNAs with the RNAi-cap technology (R-1 to R-5) and five standard siRNAs with 3´-overhangs (O-1 to O-5) were transfected at a concentration of 20 nM as to their gene silencing potency on virus gene expression in HeLa cells. The siRNAs were directed against the viral protein VP1. All siRNA with the RNAi cap (R-1 to R-5) were able to silence the VP1 gene at a concentration of 20 nM. In comparison, no silencing was observed when the siRNAs without the RNAi cap (O-1 to O-5) were used at the same concentration. As a control, ß-actin expression was detected. M, Protein marker, as indicated.
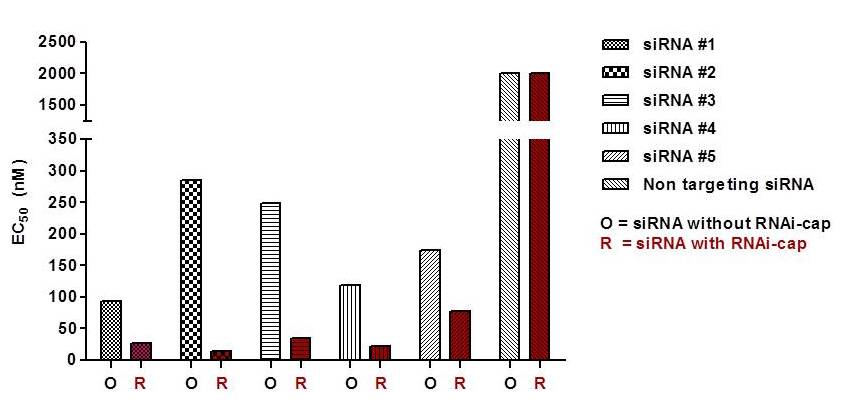
In the following experiment, cells were infected with HRV1B and cell proliferation measured. In the presence of the RNAi-cap the siRNA displayed an up to 5-fold higher potency in comparison to siRNA without the RNAi-cap. The lowest EC50 observed (siRNA#2) was 14 nM. This siRNA#2 was used in further gene knockdown experiments in animals
Fig. 3. Comparison of the potency of siRNA with or without the RNAi-cap in cell culture. Five siRNAs with the RNAi-cap technology (R) and five standard siRNAs with 3´-overhangs (O) were transfected in HeLa cells after infection with the HRV1B. The potency of the siRNA was defined as the EC50 inhibiting virus replication in cells. As a control, a non-targeting siRNA with or without the RNAi-cap was used.
B.3 The RNAi-cap results in a potent gene knockdown in vivo
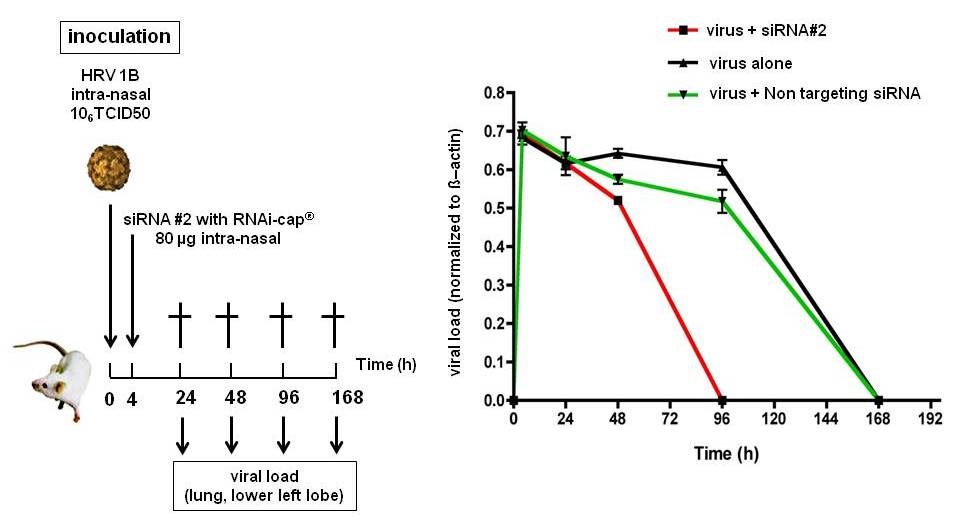
In a further step, BALB/c mice were infected with the virus HRV1B, and the siRNA applied intra-nasally at a concentration of 4 mg/kg (total amount: 80 µg, equivalent to 6 nmol of siRNA). In the animals treated (n=4), the viral load decreased at 24h, in contrast to the placebo. These results indicate that the siRNA bearing the RNAi-cap successfully cleared the virus from the lung of the animals after intra-nasal application.
Fig. 4. Inhibition of human Rhinovirus infection in VIVO by siRNA with RNAi-cap. The siRNA targeting the genome of human Rhinovirus was applied at 4 mg/kg intra-nasally in BALB/c mice. After 4 hours, a suspension of human Rhinovirus was inoculated intra-nasally, and the viral load was measured by qRT-PCR in the lower lobe of the left lung. As a negative control, siRNA with the RNAi-cap but not targeting the viral genome was used.
C. Conclusion
Riboxx has developed a new technology, the RNAi-cap, that is dedicated to gene knockdown in vivo. This new technology increases resistance of siRNA or miRNA mimics to body fluids in particular in serum up to 72 h and increases potency of gene knockdown up to 5 fold. The RNAi-cap is present in the iVORi siRNA and the CONmiR miRNA mimics of Riboxx. The siRNA and miRNA mimics used in experiments with small animals are delivered “salt free” and “endotoxin free” as CLINIgrade® products.
D. References
de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007 Jun;6(6):443-53.
Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Karikó K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008 Apr 3;452(7187):591-7.
Rohayem J, Bergmann M, Gebhardt J, Gould E, Tucker P, Mattevi A, Unge T, Hilgenfeld R, Neyts J. Antiviral strategies to control calicivirus infections. Antiviral Res. 2010 Aug;87(2):162-78.